
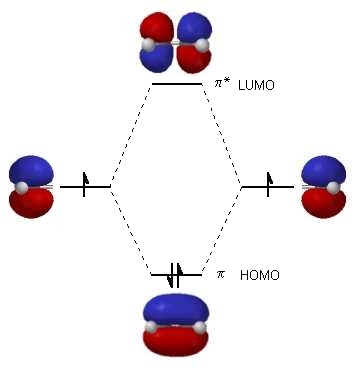
DFT method using B3LYP levels at 6-31 G(d,p) basis sets on aPentium V/ 1.6. The band gap can sometimes serve as a measure of the excitability of the molecule: the smaller the energy, the more easily a molecule's electrons will be excited. orbital (HOMO) and lowest un-occupied molecular orbital (LUMO) energies are. The difference in the HOMO's energy level and the LUMO's energy level is called the band gap. The 15th MO on the list would be called the "highest occupied molecular orbital" (HOMO) and the 16th MO on the list would be the "lowest unoccupied molecular orbital" (LUMO). For example, if a molecule has enough electrons to fill 15 MOs, the 15 MOs with the lowest energy levels will be occupied. Chemists assume that the electrons will occupy the lowest energy level MOs first. Chemists sort the molecular orbitals (MOs) by energy levels. Įach molecular orbital has a calculated energy level. One requirement for a successful HOMOLUMO interaction is that the symme-try of the HOMO must match the symmetry of the LUMO (either both symmetric or both. HOMO and LUMO are sometimes referred to as frontier orbitals. The energy difference between the HOMO and LUMO is termed the HOMO-LUMO gap. LUMO stands for lowest unoccupied molecular orbital. Walaupun terdapat beberapa perbezaan antara dua jenis ini, perbezaan utama antara Homo dan Lumo ialah Homo menyumbangkan elektron sedangkan Lumo menerima elektron. Then, by selecting the HOMO/LUMO option in the Compare mode, the database will display the HOMO of the currently selected molecule with the LUMO of the molecule chosen from the molecule comparison list.HOMO stands for highest occupied molecular orbital. Teori orbital molekul perbatasan menerangkan pembentukan orbital molekul jenis HOMO dan LUMO. An analysis of the results reveals that substituents in heteroaromatic rings of the ligands and at the amide. corresponds to electron extraction from the HOMO level and can be correlated to the ionization potential. Reaction Predict from the Optional Views menu. The consensus models predict well in 5fold crossvalidation (RMSE HOMO 0.097 eV, RMSE LUMO 0.064 eV), and on the external test sets (T1: RMSE HOMO 0.26 eV, RMSE LUMO 0.24 eV T2: RMSE HOMO 0.26 eV, RMSE LUMO 0.17 eV). Popular Answers (1) Cyclic Voltammetry The oxidation for specimen i.e. With the gap, an electron can be transferred from the HOMO to the LUMO efficiently upon photo-irradiation of the long persistent luminescence emitter. In order to compare the HOMO of one molecule with the LUMO of another, select M.O. The gap between the HOMO level and the LUMO level of the electron acceptor molecule is preferably 1.0 to 3.5 eV, more preferably 1.5 to 3.4 eV, further preferably 2.0 to 3.3 eV. If two constructive interactions are possible, the one with the lower energy difference between the HOMO and LUMO is more likely to occur. Constructive overlap is possible between the blue region of an occupied orbital and the yellow region of an unoccupied orbital or between the green region of an occupied and the red region of an unoccupied orbital. In this program, color codes for the wave function signs are arbitrary. In the regions of large overlap the orbitals (wave functions) must have the same sign. In order for bonds to form, the overlap of the orbitals must be constructive. It is often through overlap of the HOMO of one molecule with the LUMO of another that new bonds are formed during chemical reactions. All molecules have a HOMO (highest occupied molecular orbital) and a LUMO (lowest unoccupied molecular orbital).


 0 kommentar(er)
0 kommentar(er)
